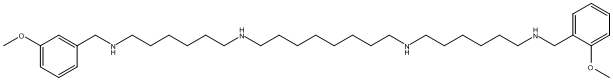
Methoctramine
Poor inhibitor of cholinesterases. Selectivity towards acetylcholinesterase over butyrylcholinesterase. AChE IC50 5.37032 microM Bolognesi et al. 2002
General
Type : Muscarinic antagonist, Not A\/B H target, Alkyl linked bis-ligand
Chemical_Nomenclature : N,N'-bis[6-[(2-methoxyphenyl)methylamino]hexyl]octane-1,8-diamine
Canonical SMILES : COC1=CC=CC=C1CNCCCCCCNCCCCCCCCNCCCCCCNCC2=CC=CC=C2OC
InChI : InChI=1S\/C36H62N4O2\/c1-41-35-23-13-11-21-33(35)31-39-29-19-9-7-17-27-37-25-15-5-3-4-6-16-26-38-28-18-8-10-20-30-40-32-34-22-12-14-24-36(34)42-2\/h11-14,21-24,37-40H,3-10,15-20,25-32H2,1-2H3
InChIKey : RPMBYDYUVKEZJA-UHFFFAOYSA-N
Other name(s) : Methoctramine free base || CHEBI:73339 || UNII-NVJ76B897D || CHEMBL27673
MW : 582.9
Formula : C36H62N4O2
CAS_number :
PubChem : 4108, 107759, 44134815, 71464675
UniChem : RPMBYDYUVKEZJA-UHFFFAOYSA-N
Target
Families : Methoctramine ligand of proteins in family
ACHE
Protein :
human-ACHE
References (59)
| Title : Balanced modulation of neuromuscular synaptic transmission via M1 and M2 muscarinic receptors during inhibition of cholinesterases - Lenina_2022_Sci.Rep_12_1688 |
| Author(s) : Lenina OA , Petrov KA |
| Ref : Sci Rep , 12 :1688 , 2022 |
| Abstract : |
| PubMedSearch : Lenina_2022_Sci.Rep_12_1688 |
| PubMedID: 35105922 |
| Title : Analgesic and preventive effects of donepezil in animal models of chemotherapy-induced peripheral neuropathy: Involvement of spinal muscarinic acetylcholine M2 receptors - Selvy_2022_Biomed.Pharmacother_149_112915 |
| Author(s) : Selvy M , Mattevi C , Dalbos C , Aissouni Y , Chapuy E , Martin PY , Collin A , Richard D , Dumontet C , Busserolles J , Conde S , Balayssac D |
| Ref : Biomed Pharmacother , 149 :112915 , 2022 |
| Abstract : |
| PubMedSearch : Selvy_2022_Biomed.Pharmacother_149_112915 |
| PubMedID: 35635358 |
| Title : Sexually diergic hypothalamic-pituitary-adrenal axis responses to selective and non-selective muscarinic antagonists prior to cholinergic stimulation by physostigmine in rats - Smail_2017_Brain.Res.Bull_137_23 |
| Author(s) : Smail MA , Soles JL , Karwoski TE , Rubin RT , Rhodes ME |
| Ref : Brain Research Bulletin , 137 :23 , 2017 |
| Abstract : |
| PubMedSearch : Smail_2017_Brain.Res.Bull_137_23 |
| PubMedID: 29122691 |
| Title : Acetylcholine induces fibrogenic effects via M2\/M3 acetylcholine receptors in non-alcoholic steatohepatitis and in primary human hepatic stellate cells - Morgan_2016_J.Gastroenterol.Hepatol_31_475 |
| Author(s) : Morgan ML , Sigala B , Soeda J , Cordero P , Nguyen V , McKee C , Mouraliderane A , Vinciguerra M , Oben JA |
| Ref : J Gastroenterol Hepatol , 31 :475 , 2016 |
| Abstract : |
| PubMedSearch : Morgan_2016_J.Gastroenterol.Hepatol_31_475 |
| PubMedID: 26270240 |
| Title : Cholinergic Neurotransmission in the Posterior Insular Cortex Is Altered in Preclinical Models of Neuropathic Pain: Key Role of Muscarinic M2 Receptors in Donepezil-Induced Antinociception - Ferrier_2015_J.Neurosci_35_16418 |
| Author(s) : Ferrier J , Bayet-Robert M , Dalmann R , El Guerrab A , Aissouni Y , Graveron-Demilly D , Chalus M , Pinguet J , Eschalier A , Richard D , Daulhac L , Marchand F , Balayssac D |
| Ref : Journal of Neuroscience , 35 :16418 , 2015 |
| Abstract : |
| PubMedSearch : Ferrier_2015_J.Neurosci_35_16418 |
| PubMedID: 26674867 |
| Title : mAChRs activation induces epithelial-mesenchymal transition on lung epithelial cells - Yang_2014_BMC.Pulm.Med_14_53 |
| Author(s) : Yang K , Song Y , Tang YB , Xu ZP , Zhou W , Hou LN , Zhu L , Yu ZH , Chen HZ , Cui YY |
| Ref : BMC Pulm Med , 14 :53 , 2014 |
| Abstract : |
| PubMedSearch : Yang_2014_BMC.Pulm.Med_14_53 |
| PubMedID: 24678619 |
| Title : Neuroanatomical basis for cholinergic modulation of locomotor networks by sacral relay neurons with ascending lumbar projections - Finkel_2014_J.Comp.Neurol_522_3437 |
| Author(s) : Finkel E , Etlin A , Cherniak M , Mor Y , Lev-Tov A , Anglister L |
| Ref : Journal of Comparative Neurology , 522 :3437 , 2014 |
| Abstract : |
| PubMedSearch : Finkel_2014_J.Comp.Neurol_522_3437 |
| PubMedID: 24752570 |
| Title : Modulation of dopaminergic neurotransmission induced by sublethal Doses of the organophosphate trichlorfon in cockroaches - Daiane_2014_Ecotoxicol.Environ.Saf_109C_56 |
| Author(s) : Daiane Stu Rmer G , de Freitas TC , de Avila Heberle M , de Assis DR , Vinade L , Batista Pereira A , Luis Franco J , Andre Dal Belo C |
| Ref : Ecotoxicology & Environmental Safety , 109C :56 , 2014 |
| Abstract : |
| PubMedSearch : Daiane_2014_Ecotoxicol.Environ.Saf_109C_56 |
| PubMedID: 25164203 |
| Title : Paradoxical neostigmine-induced TOFfade: On the role of presynaptic cholinergic and adenosine receptors - de Paula Ramos_2014_Eur.J.Pharmacol_723_389 |
| Author(s) : de Paula Ramos E , Antonio MB , Ambiel CR , Correia-de-Sa P , Alves-do-Prado W |
| Ref : European Journal of Pharmacology , 723 :389 , 2014 |
| Abstract : |
| PubMedSearch : de Paula Ramos_2014_Eur.J.Pharmacol_723_389 |
| PubMedID: 24247035 |
| Title : Endogenous ACh tonically stimulates ANP secretion in rat atria - Kim_2013_Am.J.Physiol.Heart.Circ.Physiol_305_H1050 |
| Author(s) : Kim HY , Cho KW , Xu DY , Kang DG , Lee HS |
| Ref : American Journal of Physiology Heart Circ Physiol , 305 :H1050 , 2013 |
| Abstract : |
| PubMedSearch : Kim_2013_Am.J.Physiol.Heart.Circ.Physiol_305_H1050 |
| PubMedID: 23913708 |
| Title : Cholinergic modulation of primary afferent glutamatergic transmission in rat medullary dorsal horn neurons - Jeong_2013_Neuropharmacol_75C_295 |
| Author(s) : Jeong SG , Choi IS , Cho JH , Jang IS |
| Ref : Neuropharmacology , 75C :295 , 2013 |
| Abstract : |
| PubMedSearch : Jeong_2013_Neuropharmacol_75C_295 |
| PubMedID: 23954675 |
| Title : GRK5 deficiency accelerates {beta}-amyloid accumulation in Tg2576 mice via impaired cholinergic activity - Cheng_2010_J.Biol.Chem_285_41541 |
| Author(s) : Cheng S , Li L , He S , Liu J , Sun Y , He M , Grasing K , Premont RT , Suo WZ |
| Ref : Journal of Biological Chemistry , 285 :41541 , 2010 |
| Abstract : |
| PubMedSearch : Cheng_2010_J.Biol.Chem_285_41541 |
| PubMedID: 21041302 |
| Title : Effects of cholinesterase inhibition in supraspinal and spinal neural pathways on the micturition reflex in rats - Masuda_2009_BJU.Int_104_1163 |
| Author(s) : Masuda H , Chancellor MB , Kihara K , Sakai Y , Koga F , Azuma H , De Groat WC , Yoshimura N |
| Ref : BJU Int , 104 :1163 , 2009 |
| Abstract : |
| PubMedSearch : Masuda_2009_BJU.Int_104_1163 |
| PubMedID: 19338542 |
| Title : Effects of estrogen on intracellular signaling pathways linked to activation of muscarinic acetylcholine receptors and on acetylcholinesterase activity in rat hippocampus - Pereira_2008_Biochem.Pharmacol_75_1827 |
| Author(s) : Pereira RT , Porto CS , Godinho RO , Abdalla FM |
| Ref : Biochemical Pharmacology , 75 :1827 , 2008 |
| Abstract : |
| PubMedSearch : Pereira_2008_Biochem.Pharmacol_75_1827 |
| PubMedID: 18329631 |
| Title : Muscarinic receptor-independent activation of cyclic adenosine monophosphate-dependent protein kinase in rostral ventrolateral medulla underlies the sympathoexcitatory phase of cardiovascular responses during mevinphos intoxication in the rat - Tsai_2007_Shock_27_559 |
| Author(s) : Tsai CY , Wu CH , Chan SH , Chang AY |
| Ref : Shock , 27 :559 , 2007 |
| Abstract : |
| PubMedSearch : Tsai_2007_Shock_27_559 |
| PubMedID: 17438462 |
| Title : Pharmacological characterization of muscarinic receptor subtypes mediating vasoconstriction of human umbilical vein - Pujol Lereis_2006_Br.J.Pharmacol_147_516 |
| Author(s) : Pujol Lereis VA , Hita FJ , Gobbi MD , Verdi MG , Rodriguez MC , Rothlin RP |
| Ref : British Journal of Pharmacology , 147 :516 , 2006 |
| Abstract : |
| PubMedSearch : Pujol Lereis_2006_Br.J.Pharmacol_147_516 |
| PubMedID: 16444291 |
| Title : Spinal alpha(2)-adrenergic and muscarinic receptors and the NO release cascade mediate supraspinally produced effectiveness of gabapentin at decreasing mechanical hypersensitivity in mice after partial nerve injury - Takasu_2006_Br.J.Pharmacol_148_233 |
| Author(s) : Takasu K , Honda M , Ono H , Tanabe M |
| Ref : British Journal of Pharmacology , 148 :233 , 2006 |
| Abstract : |
| PubMedSearch : Takasu_2006_Br.J.Pharmacol_148_233 |
| PubMedID: 16582934 |
| Title : Depolarization initiates phasic acetylcholine release by relief of a tonic block imposed by presynaptic M2 muscarinic receptors - Parnas_2005_J.Neurophysiol_93_3257 |
| Author(s) : Parnas H , Slutsky I , Rashkovan G , Silman I , Wess J , Parnas I |
| Ref : Journal of Neurophysiology , 93 :3257 , 2005 |
| Abstract : |
| PubMedSearch : Parnas_2005_J.Neurophysiol_93_3257 |
| PubMedID: 15703226 |
| Title : Cholinergic inhibition of neocortical pyramidal neurons - Gulledge_2005_J.Neurosci_25_10308 |
| Author(s) : Gulledge AT , Stuart GJ |
| Ref : Journal of Neuroscience , 25 :10308 , 2005 |
| Abstract : |
| PubMedSearch : Gulledge_2005_J.Neurosci_25_10308 |
| PubMedID: 16267239 |
| Title : Intrathecal neostigmine reduces the zymosan-induced inflammatory response in a mouse air pouch model via adrenomedullary activity: involvement of spinal muscarinic type 2 receptors - Yoon_2005_Neuropharmacol_49_275 |
| Author(s) : Yoon SY , Kwon YB , Kim HW , Roh DH , Kang SY , Kim CY , Han HJ , Kim KW , Yang IS , Beitz AJ , Lee JH |
| Ref : Neuropharmacology , 49 :275 , 2005 |
| Abstract : |
| PubMedSearch : Yoon_2005_Neuropharmacol_49_275 |
| PubMedID: 15922370 |
| Title : M2 muscarinic receptors in pontine reticular formation of C57BL\/6J mouse contribute to rapid eye movement sleep generation - Coleman_2004_Neurosci_126_821 |
| Author(s) : Coleman CG , Lydic R , Baghdoyan HA |
| Ref : Neuroscience , 126 :821 , 2004 |
| Abstract : |
| PubMedSearch : Coleman_2004_Neurosci_126_821 |
| PubMedID: 15207317 |
| Title : 3H-Noradrenaline release from mouse iris-ciliary body: role of presynaptic muscarinic heteroreceptors - Bernhard_2004_Naunyn.Schmiedebergs.Arch.Pharmacol_370_305 |
| Author(s) : Bernhard M , Takeda K , Keller C , Haslebacher M , Lambrou GN , Trendelenburg AU |
| Ref : Naunyn Schmiedebergs Arch Pharmacol , 370 :305 , 2004 |
| Abstract : |
| PubMedSearch : Bernhard_2004_Naunyn.Schmiedebergs.Arch.Pharmacol_370_305 |
| PubMedID: 15375642 |
| Title : Polymethylene tetraamine backbone as template for the development of biologically active polyamines - Melchiorre_2003_Med.Res.Rev_23_200 |
| Author(s) : Melchiorre C , Antonello A , Banzi R , Bolognesi ML , Minarini A , Rosini M , Tumiatti V |
| Ref : Med Res Rev , 23 :200 , 2003 |
| Abstract : |
| PubMedSearch : Melchiorre_2003_Med.Res.Rev_23_200 |
| PubMedID: 12500289 |
| Title : Characteristics of changes in cholinergic function and impairment of learning and memory-related behavior induced by olfactory bulbectomy - Hozumi_2003_Behav.Brain.Res_138_9 |
| Author(s) : Hozumi S , Nakagawasai O , Tan-No K , Niijima F , Yamadera F , Murata A , Arai Y , Yasuhara H , Tadano T |
| Ref : Behavioural Brain Research , 138 :9 , 2003 |
| Abstract : |
| PubMedSearch : Hozumi_2003_Behav.Brain.Res_138_9 |
| PubMedID: 12493626 |
| Title : Heterogeneity of presynaptic muscarinic receptors mediating inhibition of sympathetic transmitter release: a study with M2- and M4-receptor-deficient mice - Trendelenburg_2003_Br.J.Pharmacol_138_469 |
| Author(s) : Trendelenburg AU , Gomeza J , Klebroff W , Zhou H , Wess J |
| Ref : British Journal of Pharmacology , 138 :469 , 2003 |
| Abstract : |
| PubMedSearch : Trendelenburg_2003_Br.J.Pharmacol_138_469 |
| PubMedID: 12569072 |
| Title : Role of mitogen-activated protein kinase pathway in acetylcholine-mediated in vitro relaxation of rat pulmonary artery - Choy_2002_Eur.J.Pharmacol_434_55 |
| Author(s) : Choy WY , Wong YF , Kwan YW , Au AL , Lau WH , Raymond K , Zuo JZ |
| Ref : European Journal of Pharmacology , 434 :55 , 2002 |
| Abstract : |
| PubMedSearch : Choy_2002_Eur.J.Pharmacol_434_55 |
| PubMedID: 11755166 |
| Title : Structure-activity relationships of methoctramine-related polyamines as muscular nicotinic receptor noncompetitive antagonists. 3. Effect of inserting the tetraamine backbone into a macrocyclic structure - Bolognesi_2002_J.Med.Chem_45_3286 |
| Author(s) : Bolognesi ML , Bixel MG , Marucci G , Bartolini M , Krauss M , Angeli P , Antonello A , Rosini M , Tumiatti V , Hucho F , Melchiorre C |
| Ref : Journal of Medicinal Chemistry , 45 :3286 , 2002 |
| Abstract : |
| PubMedSearch : Bolognesi_2002_J.Med.Chem_45_3286 |
| PubMedID: 12109912 |
| Title : Regulation of acetylcholine release by muscarinic receptors at the mouse neuromuscular junction depends on the activity of acetylcholinesterase - Minic_2002_Eur.J.Neurosci_15_439 |
| Author(s) : Minic J , Molgo J , Karlsson E , Krejci E |
| Ref : European Journal of Neuroscience , 15 :439 , 2002 |
| Abstract : |
| PubMedSearch : Minic_2002_Eur.J.Neurosci_15_439 |
| PubMedID: 11876771 |
| Title : Presynaptic M(2) muscarinic receptors are involved in controlling the kinetics of ACh release at the frog neuromuscular junction - Slutsky_2001_J.Physiol_536_717 |
| Author(s) : Slutsky I , Silman I , Parnas I , Parnas H |
| Ref : The Journal of Physiology , 536 :717 , 2001 |
| Abstract : |
| PubMedSearch : Slutsky_2001_J.Physiol_536_717 |
| PubMedID: 11691867 |
| Title : Pharmacological identification of acetylcholine receptor subtypes in echinoderm smooth muscle (Sclerodactyla briareus) - Devlin_2000_Comp.Biochem.Physiol.C.Toxicol.Pharmacol_125_53 |
| Author(s) : Devlin CL , Schlosser W , Belz DT , Kodiak K , Nash RF , Zitomer N |
| Ref : Comparative Biochemistry & Physiology C Toxicol Pharmacol , 125 :53 , 2000 |
| Abstract : |
| PubMedSearch : Devlin_2000_Comp.Biochem.Physiol.C.Toxicol.Pharmacol_125_53 |
| PubMedID: 11790330 |
| Title : Inhibition of GTPase activity of Gi proteins and decreased agonist affinity at M2 muscarinic acetylcholine receptors by spermine and methoctramine - Daeffler_1999_Br.J.Pharmacol_127_1021 |
| Author(s) : Daeffler L , Chahdi A , Gies JP , Landry Y |
| Ref : British Journal of Pharmacology , 127 :1021 , 1999 |
| Abstract : |
| PubMedSearch : Daeffler_1999_Br.J.Pharmacol_127_1021 |
| PubMedID: 10433511 |
| Title : Design, synthesis, and biological evaluation of symmetrically and unsymmetrically substituted methoctramine-related polyamines as muscular nicotinic receptor noncompetitive antagonists - Rosini_1999_J.Med.Chem_42_5212 |
| Author(s) : Rosini M , Budriesi R , Bixel MG , Bolognesi ML , Chiarini A , Hucho F , Krogsgaard-Larsen P , Mellor IR , Minarini A , Tumiatti V , Usherwood PN , Melchiorre C |
| Ref : Journal of Medicinal Chemistry , 42 :5212 , 1999 |
| Abstract : |
| PubMedSearch : Rosini_1999_J.Med.Chem_42_5212 |
| PubMedID: 10602706 |
| Title : Muscarinic receptor modulation of GABA-mediated giant depolarizing potentials in the neonatal rat hippocampus - Avignone_1999_J.Physiol_518_97 |
| Author(s) : Avignone E , Cherubini E |
| Ref : The Journal of Physiology , 518 :97 , 1999 |
| Abstract : |
| PubMedSearch : Avignone_1999_J.Physiol_518_97 |
| PubMedID: 10373692 |
| Title : The antiallodynic effects of intrathecal cholinesterase inhibitors in a rat model of neuropathic pain - Hwang_1999_Anesthesiology_90_492 |
| Author(s) : Hwang JH , Hwang KS , Leem JK , Park PH , Han SM , Lee DM |
| Ref : Anesthesiology , 90 :492 , 1999 |
| Abstract : |
| PubMedSearch : Hwang_1999_Anesthesiology_90_492 |
| PubMedID: 9952157 |
| Title : The M2 muscarinic receptor antagonist methoctramine activates mast cells via pertussis toxin-sensitive G proteins - Chahdi_1998_Naunyn.Schmiedebergs.Arch.Pharmacol_357_357 |
| Author(s) : Chahdi A , Daeffler L , Bueb JL , Gies JP , Landry Y |
| Ref : Naunyn Schmiedebergs Arch Pharmacol , 357 :357 , 1998 |
| Abstract : |
| PubMedSearch : Chahdi_1998_Naunyn.Schmiedebergs.Arch.Pharmacol_357_357 |
| PubMedID: 9606019 |
| Title : Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn - Baba_1998_J.Physiol_508_83 |
| Author(s) : Baba H , Kohno T , Okamoto M , Goldstein PA , Shimoji K , Yoshimura M |
| Ref : The Journal of Physiology , 508 :83 , 1998 |
| Abstract : |
| PubMedSearch : Baba_1998_J.Physiol_508_83 |
| PubMedID: 9490821 |
| Title : Pressor and bradycardic effects of tacrine and other acetylcholinesterase inhibitors in the rat - Lazartigues_1998_Eur.J.Pharmacol_361_61 |
| Author(s) : Lazartigues E , Freslon JL , Tellioglu T , Brefel-Courbon C , Pelat M , Tran MA , Montastruc JL , Rascol O |
| Ref : European Journal of Pharmacology , 361 :61 , 1998 |
| Abstract : |
| PubMedSearch : Lazartigues_1998_Eur.J.Pharmacol_361_61 |
| PubMedID: 9851542 |
| Title : Age-dependent muscarinic stimulation of beta-endorphin secretion from rat neurointermediate lobe in vitro - Acs_1997_Brain.Res.Bull_44_719 |
| Author(s) : Acs Z , Barna I , Koenig JI , Makara GB |
| Ref : Brain Research Bulletin , 44 :719 , 1997 |
| Abstract : |
| PubMedSearch : Acs_1997_Brain.Res.Bull_44_719 |
| PubMedID: 9421136 |
| Title : Poster: Muscarinic antagonists methoctramine and HHsiD can inhibite G-protein activity - |
| Author(s) : Daeffler L , Van Gelderen JG , Landry Y , Gies JP |
| Ref : Life Sciences , 60 :1178 , 1997 |
| PubMedID: |
| Title : Acetylcholinesterase histochemistry and functional characterization of the muscarinic receptor mediating the contraction of the bovine oesophageal groove - Barahona_1997_J.Auton.Pharmacol_17_77 |
| Author(s) : Barahona MV , Sanchez-Fortun S , San Andres MD , Ballesteros E , San Andres M |
| Ref : J Auton Pharmacol , 17 :77 , 1997 |
| Abstract : |
| PubMedSearch : Barahona_1997_J.Auton.Pharmacol_17_77 |
| PubMedID: 9234077 |
| Title : M4 muscarinic autoreceptor-mediated inhibition of -3H-acetylcholine release in the rat isolated urinary bladder - D'Agostino_1997_J.Pharmacol.Exp.Ther_283_750 |
| Author(s) : D'Agostino G , Barbieri A , Chiossa E , Tonini M |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 283 :750 , 1997 |
| Abstract : |
| PubMedSearch : D'Agostino_1997_J.Pharmacol.Exp.Ther_283_750 |
| PubMedID: 9353395 |
| Title : The design of novel methoctramine-related tetraamines as muscarinic receptor subtype selective antagonists - Melchiorre_1995_Life.Sci_56(11-12)_837 |
| Author(s) : Melchiorre C , Minarini A , Budriesi R , Chiarini A , Spampinato S , Tumiatti V |
| Ref : Life Sciences , 56(11-12) :837 , 1995 |
| Abstract : |
| PubMedSearch : Melchiorre_1995_Life.Sci_56(11-12)_837 |
| PubMedID: 10188783 |
| Title : Mediation by M3-muscarinic receptors of both endothelium-dependent contraction and relaxation to acetylcholine in the aorta of the spontaneously hypertensive rat - Boulanger_1994_Br.J.Pharmacol_112_519 |
| Author(s) : Boulanger CM , Morrison KJ , Vanhoutte PM |
| Ref : British Journal of Pharmacology , 112 :519 , 1994 |
| Abstract : |
| PubMedSearch : Boulanger_1994_Br.J.Pharmacol_112_519 |
| PubMedID: 8075871 |
| Title : Muscarinic stimulation promotes cultured Purkinje cell survival: a role for acetylcholine in cerebellar development? - Mount_1994_J.Neurochem_63_2065 |
| Author(s) : Mount HT , Dreyfus CF , Black IB |
| Ref : Journal of Neurochemistry , 63 :2065 , 1994 |
| Abstract : |
| PubMedSearch : Mount_1994_J.Neurochem_63_2065 |
| PubMedID: 7964724 |
| Title : A unique central cholinergic deficit in the spontaneously hypertensive rat: physostigmine reveals a bradycardia associated with sensory stimulation - Taylor_1994_J.Pharmacol.Exp.Ther_268_1081 |
| Author(s) : Taylor BK , Holloway D , Printz MP |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 268 :1081 , 1994 |
| Abstract : |
| PubMedSearch : Taylor_1994_J.Pharmacol.Exp.Ther_268_1081 |
| PubMedID: 8138921 |
| Title : Intrathecal cholinergic agonists lessen bupivacaine spinal-block-induced hypotension in rats - Carp_1994_Anesth.Analg_79_112 |
| Author(s) : Carp H , Jayaram A , Morrow D |
| Ref : Anesthesia & Analgesia , 79 :112 , 1994 |
| Abstract : |
| PubMedSearch : Carp_1994_Anesth.Analg_79_112 |
| PubMedID: 8010419 |
| Title : Central muscarinic M2 cholinoceptors involved in cholinergic hypertension - Ozkutlu_1993_Eur.J.Pharmacol_250_349 |
| Author(s) : Ozkutlu U , Onat F , Aslan AN , Oktay S |
| Ref : European Journal of Pharmacology , 250 :349 , 1993 |
| Abstract : |
| PubMedSearch : Ozkutlu_1993_Eur.J.Pharmacol_250_349 |
| PubMedID: 8112394 |
| Title : Actions of methoctramine, a muscarinic M2 receptor antagonist, on muscarinic and nicotinic cholinoceptors in guinea-pig airways in vivo and in vitro - Watson_1992_Br.J.Pharmacol_105_107 |
| Author(s) : Watson N , Barnes PJ , Maclagan J |
| Ref : British Journal of Pharmacology , 105 :107 , 1992 |
| Abstract : |
| PubMedSearch : Watson_1992_Br.J.Pharmacol_105_107 |
| PubMedID: 1596672 |
| Title : The autoinhibitory feedback control of acetylcholine release in human neocortex tissue - Feuerstein_1992_Brain.Res_572_64 |
| Author(s) : Feuerstein TJ , Lehmann J , Sauermann W , van Velthoven V , Jackisch R |
| Ref : Brain Research , 572 :64 , 1992 |
| Abstract : |
| PubMedSearch : Feuerstein_1992_Brain.Res_572_64 |
| PubMedID: 1611539 |
| Title : Presynaptic effects of methoctramine on release of acetylcholine - Torocsik_1991_Neuropharmacol_30_293 |
| Author(s) : Torocsik A , Vizi ES |
| Ref : Neuropharmacology , 30 :293 , 1991 |
| Abstract : |
| PubMedSearch : Torocsik_1991_Neuropharmacol_30_293 |
| PubMedID: 1852264 |
| Title : Poster: Analgesia induced by the M2 antagonist methoctramine administered i.c.v. - |
| Author(s) : Gualtieri F , Ghelardini C , Giotti A , Malcangio M , Malmberg-Aiello P , Bartolini A |
| Ref : Trends in Pharmacological Sciences , Suppl :99 , 1989 |
| PubMedID: |
| Title : Methoctramine, a cardioselective muscarinic antagonist, stimulates phosphoinositide hydrolysis in rat cerebral cortex - Lee_1989_Eur.J.Pharmacol_167_295 |
| Author(s) : Lee NH , Forray C , El-Fakahany EE |
| Ref : European Journal of Pharmacology , 167 :295 , 1989 |
| Abstract : |
| PubMedSearch : Lee_1989_Eur.J.Pharmacol_167_295 |
| PubMedID: 2556288 |
| Title : Different mechanisms of antagonism by methoctramine of two neuronal muscarinic receptor-mediated second messenger responses - Lee_1989_J.Pharmacol.Exp.Ther_251_992 |
| Author(s) : Lee NH , Fryer AD , Forray C , El-Fakahany EE |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 251 :992 , 1989 |
| Abstract : |
| PubMedSearch : Lee_1989_J.Pharmacol.Exp.Ther_251_992 |
| PubMedID: 2557423 |
| Title : Methoctramine, a polymethylene tetraamine, differentiates three subtypes of muscarinic receptor in direct binding studies - Michel_1988_Eur.J.Pharmacol_145_61 |
| Author(s) : Michel AD , Whiting RL |
| Ref : European Journal of Pharmacology , 145 :61 , 1988 |
| Abstract : |
| PubMedSearch : Michel_1988_Eur.J.Pharmacol_145_61 |
| PubMedID: 3127224 |
| Title : Methoctramine reveals heterogeneity of M2 muscarinic receptors in longitudinal ileal smooth muscle membranes - Michel_1988_Eur.J.Pharmacol_145_305 |
| Author(s) : Michel AD , Whiting RL |
| Ref : European Journal of Pharmacology , 145 :305 , 1988 |
| Abstract : |
| PubMedSearch : Michel_1988_Eur.J.Pharmacol_145_305 |
| PubMedID: 3350049 |
| Title : The interaction of methoctramine and himbacine at atrial, smooth muscle and endothelial muscarinic receptors in vitro - Eglen_1988_Br.J.Pharmacol_95_1031 |
| Author(s) : Eglen RM , Montgomery WW , Dainty IA , Dubuque LK , Whiting RL |
| Ref : British Journal of Pharmacology , 95 :1031 , 1988 |
| Abstract : |
| PubMedSearch : Eglen_1988_Br.J.Pharmacol_95_1031 |
| PubMedID: 3219478 |
| Title : Binding and functional characterization of the cardioselective muscarinic antagonist methoctramine - Giraldo_1988_J.Pharmacol.Exp.Ther_244_1016 |
| Author(s) : Giraldo E , Micheletti R , Montagna E , Giachetti A , Vigano MA , Ladinsky H , Melchiorre C |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 244 :1016 , 1988 |
| Abstract : |
| PubMedSearch : Giraldo_1988_J.Pharmacol.Exp.Ther_244_1016 |
| PubMedID: 3252019 |
| Title : Methoctramine selectively blocks cardiac muscarinic M2 receptors in vivo - Wess_1988_Naunyn.Schmiedebergs.Arch.Pharmacol_338_246 |
| Author(s) : Wess J , Angeli P , Melchiorre C , Moser U , Mutschler E , Lambrecht G |
| Ref : Naunyn Schmiedebergs Arch Pharmacol , 338 :246 , 1988 |
| Abstract : |
| PubMedSearch : Wess_1988_Naunyn.Schmiedebergs.Arch.Pharmacol_338_246 |
| PubMedID: 3057387 |
| Title : Antimuscarinic action of methoctramine, a new cardioselective M-2 muscarinic receptor antagonist, alone and in combination with atropine and gallamine - Melchiorre_1987_Eur.J.Pharmacol_144_117 |
| Author(s) : Melchiorre C , Angeli P , Lambrecht G , Mutschler E , Picchio MT , Wess J |
| Ref : European Journal of Pharmacology , 144 :117 , 1987 |
| Abstract : |
| PubMedSearch : Melchiorre_1987_Eur.J.Pharmacol_144_117 |
| PubMedID: 3436364 |