Propidium
The most common use of propidium is because it is a membrane impermeable DNA intercalator. Has red fluorescence at 488 nm. Has the following spectral properties: extinction coefficient: 5,900 M -1 cm -1 (493 nm); 55,700 M -1 cm -1 (287 nm) 13,800 M -1 cm -1 (252.5 nm); excitation maximum: 536 nm; emission maximum: 617 nm
General
Type : Phenanthridinium, Fluorescent Probe
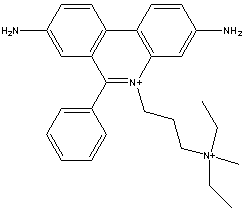
Chemical_Nomenclature : 3,8-diamino-5[3-(diethylmethylammonio)propyl]-6-phenylphenanthridinium diiodide
Canonical SMILES : CC[N+](C)(CC)CCC[N+]1=C2C=C(C=CC2=C3C=CC(=CC3=C1C4=CC=CC=C4)N)N
InChI : InChI=1S\/C27H33N4\/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20\/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3\/q+1\/p+1
InChIKey : ZDWVWKDAWBGPDN-UHFFFAOYSA-O
Other name(s) : 36015-30-2 || UNII-74NX23J96Y || CHEBI:51246 || 74NX23J96Y || CHEMBL345124 || SCHEMBL55279 || ZINC622264 || PRM
MW : 668.4
Formula : C27H34N4I2
CAS_number : 25535-16-4
PubChem : 4939
UniChem : ZDWVWKDAWBGPDN-UHFFFAOYSA-O
Wikipedia : Propidium_iodide
Target
Families : Propidium ligand of proteins in family
ACHE
BCHE
Protein :
human-BCHE
mouse-ACHE
References (57)
| Title : Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study - Rosenberry_2017_Molecules_22_ |
| Author(s) : Rosenberry TL , Brazzolotto X , Macdonald IR , Wandhammer M , Trovaslet-Leroy M , Darvesh S , Nachon F |
| Ref : Molecules , 22 : , 2017 |
| Abstract : |
| PubMedSearch : Rosenberry_2017_Molecules_22_ |
| PubMedID: 29186056 |
| Gene_locus related to this paper: human-BCHE |
| Title : Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study - Rosenberry_2017_Molecules_22_ |
| Author(s) : Rosenberry TL , Brazzolotto X , Macdonald IR , Wandhammer M , Trovaslet-Leroy M , Darvesh S , Nachon F |
| Ref : Molecules , 22 : , 2017 |
| Abstract : |
| PubMedSearch : Rosenberry_2017_Molecules_22_ |
| PubMedID: 29186056 |
| Gene_locus related to this paper: human-BCHE |
| Title : Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study - Rosenberry_2017_Molecules_22_ |
| Author(s) : Rosenberry TL , Brazzolotto X , Macdonald IR , Wandhammer M , Trovaslet-Leroy M , Darvesh S , Nachon F |
| Ref : Molecules , 22 : , 2017 |
| Abstract : |
| PubMedSearch : Rosenberry_2017_Molecules_22_ |
| PubMedID: 29186056 |
| Gene_locus related to this paper: human-BCHE |
| Title : beta-Amyloid aggregation induced by human acetylcholinesterase: inhibition studies - Bartolini_2003_Biochem.Pharmacol_65_407 |
| Author(s) : Bartolini M , Bertucci C , Cavrini V , Andrisano V |
| Ref : Biochemical Pharmacology , 65 :407 , 2003 |
| Abstract : |
| PubMedSearch : Bartolini_2003_Biochem.Pharmacol_65_407 |
| PubMedID: 12527333 |
| Title : beta-Amyloid aggregation induced by human acetylcholinesterase: inhibition studies - Bartolini_2003_Biochem.Pharmacol_65_407 |
| Author(s) : Bartolini M , Bertucci C , Cavrini V , Andrisano V |
| Ref : Biochemical Pharmacology , 65 :407 , 2003 |
| Abstract : |
| PubMedSearch : Bartolini_2003_Biochem.Pharmacol_65_407 |
| PubMedID: 12527333 |
| Title : beta-Amyloid aggregation induced by human acetylcholinesterase: inhibition studies - Bartolini_2003_Biochem.Pharmacol_65_407 |
| Author(s) : Bartolini M , Bertucci C , Cavrini V , Andrisano V |
| Ref : Biochemical Pharmacology , 65 :407 , 2003 |
| Abstract : |
| PubMedSearch : Bartolini_2003_Biochem.Pharmacol_65_407 |
| PubMedID: 12527333 |
| Title : A steric blockade model for inhibition of acetylcholinesterase by peripheral site ligands and substrate - Rosenberry_1999_Chem.Biol.Interact_119-120_85 |
| Author(s) : Rosenberry TL , Mallender WD , Thomas PJ , Szegletes T |
| Ref : Chemico-Biological Interactions , 119-120 :85 , 1999 |
| Abstract : |
| PubMedSearch : Rosenberry_1999_Chem.Biol.Interact_119-120_85 |
| PubMedID: 10421442 |
| Title : A steric blockade model for inhibition of acetylcholinesterase by peripheral site ligands and substrate - Rosenberry_1999_Chem.Biol.Interact_119-120_85 |
| Author(s) : Rosenberry TL , Mallender WD , Thomas PJ , Szegletes T |
| Ref : Chemico-Biological Interactions , 119-120 :85 , 1999 |
| Abstract : |
| PubMedSearch : Rosenberry_1999_Chem.Biol.Interact_119-120_85 |
| PubMedID: 10421442 |
| Title : The influence of peripheral site ligands on the reaction of symmetric and chiral organophosphates with wildtype and mutant acetylcholinesterases - Radic_1999_Chem.Biol.Interact_119-120_111 |
| Author(s) : Radic Z , Taylor P |
| Ref : Chemico-Biological Interactions , 119-120 :111 , 1999 |
| Abstract : |
| PubMedSearch : Radic_1999_Chem.Biol.Interact_119-120_111 |
| PubMedID: 10421444 |
| Title : A steric blockade model for inhibition of acetylcholinesterase by peripheral site ligands and substrate - Rosenberry_1999_Chem.Biol.Interact_119-120_85 |
| Author(s) : Rosenberry TL , Mallender WD , Thomas PJ , Szegletes T |
| Ref : Chemico-Biological Interactions , 119-120 :85 , 1999 |
| Abstract : |
| PubMedSearch : Rosenberry_1999_Chem.Biol.Interact_119-120_85 |
| PubMedID: 10421442 |
| Title : The influence of peripheral site ligands on the reaction of symmetric and chiral organophosphates with wildtype and mutant acetylcholinesterases - Radic_1999_Chem.Biol.Interact_119-120_111 |
| Author(s) : Radic Z , Taylor P |
| Ref : Chemico-Biological Interactions , 119-120 :111 , 1999 |
| Abstract : |
| PubMedSearch : Radic_1999_Chem.Biol.Interact_119-120_111 |
| PubMedID: 10421444 |
| Title : The influence of peripheral site ligands on the reaction of symmetric and chiral organophosphates with wildtype and mutant acetylcholinesterases - Radic_1999_Chem.Biol.Interact_119-120_111 |
| Author(s) : Radic Z , Taylor P |
| Ref : Chemico-Biological Interactions , 119-120 :111 , 1999 |
| Abstract : |
| PubMedSearch : Radic_1999_Chem.Biol.Interact_119-120_111 |
| PubMedID: 10421444 |
| Title : Nonequilibrium analysis alters the mechanistic interpretation of inhibition of acetylcholinesterase by peripheral site ligands - Szegletes_1998_Biochemistry_37_4206 |
| Author(s) : Szegletes T , Mallender WD , Rosenberry TL |
| Ref : Biochemistry , 37 :4206 , 1998 |
| Abstract : |
| PubMedSearch : Szegletes_1998_Biochemistry_37_4206 |
| PubMedID: 9521743 |
| Title : Nonequilibrium analysis alters the mechanistic interpretation of inhibition of acetylcholinesterase by peripheral site ligands - Szegletes_1998_Biochemistry_37_4206 |
| Author(s) : Szegletes T , Mallender WD , Rosenberry TL |
| Ref : Biochemistry , 37 :4206 , 1998 |
| Abstract : |
| PubMedSearch : Szegletes_1998_Biochemistry_37_4206 |
| PubMedID: 9521743 |
| Title : Nonequilibrium analysis alters the mechanistic interpretation of inhibition of acetylcholinesterase by peripheral site ligands - Szegletes_1998_Biochemistry_37_4206 |
| Author(s) : Szegletes T , Mallender WD , Rosenberry TL |
| Ref : Biochemistry , 37 :4206 , 1998 |
| Abstract : |
| PubMedSearch : Szegletes_1998_Biochemistry_37_4206 |
| PubMedID: 9521743 |
| Title : Binding of the neurotoxin fasciculin 2 to the acetylcholinesterase peripheral site drastically reduces the association and dissociation rate constants for N-methylacridinium binding to the active site - Rosenberry_1996_Biochemistry_35_685 |
| Author(s) : Rosenberry TL , Rabl CR , Neumann E |
| Ref : Biochemistry , 35 :685 , 1996 |
| Abstract : |
| PubMedSearch : Rosenberry_1996_Biochemistry_35_685 |
| PubMedID: 8547248 |
| Title : Asp7O in the peripheral anionic site of human butyrylcholinesterase - Masson_1996_Eur.J.Biochem_235_36 |
| Author(s) : Masson P , Froment MT , Bartels CF , Lockridge O |
| Ref : European Journal of Biochemistry , 235 :36 , 1996 |
| Abstract : |
| PubMedSearch : Masson_1996_Eur.J.Biochem_235_36 |
| PubMedID: 8631355 |
| Title : Cloning and expression of acetylcholinesterase from Bungarus fasciatus venom. A new type of cooh-terminal domain\; involvement of a positively charged residue in the peripheral site - Cousin_1996_J.Biol.Chem_271_15099 |
| Author(s) : Cousin X , Bon S , Duval N , Massoulie J , Bon C |
| Ref : Journal of Biological Chemistry , 271 :15099 , 1996 |
| Abstract : |
| PubMedSearch : Cousin_1996_J.Biol.Chem_271_15099 |
| PubMedID: 8662867 |
| Gene_locus related to this paper: bunfa-ACHE |
| Title : Cloning and expression of acetylcholinesterase from Bungarus fasciatus venom. A new type of cooh-terminal domain\; involvement of a positively charged residue in the peripheral site - Cousin_1996_J.Biol.Chem_271_15099 |
| Author(s) : Cousin X , Bon S , Duval N , Massoulie J , Bon C |
| Ref : Journal of Biological Chemistry , 271 :15099 , 1996 |
| Abstract : |
| PubMedSearch : Cousin_1996_J.Biol.Chem_271_15099 |
| PubMedID: 8662867 |
| Gene_locus related to this paper: bunfa-ACHE |
| Title : Cloning and expression of acetylcholinesterase from Bungarus fasciatus venom. A new type of cooh-terminal domain\; involvement of a positively charged residue in the peripheral site - Cousin_1996_J.Biol.Chem_271_15099 |
| Author(s) : Cousin X , Bon S , Duval N , Massoulie J , Bon C |
| Ref : Journal of Biological Chemistry , 271 :15099 , 1996 |
| Abstract : |
| PubMedSearch : Cousin_1996_J.Biol.Chem_271_15099 |
| PubMedID: 8662867 |
| Gene_locus related to this paper: bunfa-ACHE |
| Title : Asp7O in the peripheral anionic site of human butyrylcholinesterase - Masson_1996_Eur.J.Biochem_235_36 |
| Author(s) : Masson P , Froment MT , Bartels CF , Lockridge O |
| Ref : European Journal of Biochemistry , 235 :36 , 1996 |
| Abstract : |
| PubMedSearch : Masson_1996_Eur.J.Biochem_235_36 |
| PubMedID: 8631355 |
| Title : Binding of the neurotoxin fasciculin 2 to the acetylcholinesterase peripheral site drastically reduces the association and dissociation rate constants for N-methylacridinium binding to the active site - Rosenberry_1996_Biochemistry_35_685 |
| Author(s) : Rosenberry TL , Rabl CR , Neumann E |
| Ref : Biochemistry , 35 :685 , 1996 |
| Abstract : |
| PubMedSearch : Rosenberry_1996_Biochemistry_35_685 |
| PubMedID: 8547248 |
| Title : Asp7O in the peripheral anionic site of human butyrylcholinesterase - Masson_1996_Eur.J.Biochem_235_36 |
| Author(s) : Masson P , Froment MT , Bartels CF , Lockridge O |
| Ref : European Journal of Biochemistry , 235 :36 , 1996 |
| Abstract : |
| PubMedSearch : Masson_1996_Eur.J.Biochem_235_36 |
| PubMedID: 8631355 |
| Title : Binding of the neurotoxin fasciculin 2 to the acetylcholinesterase peripheral site drastically reduces the association and dissociation rate constants for N-methylacridinium binding to the active site - Rosenberry_1996_Biochemistry_35_685 |
| Author(s) : Rosenberry TL , Rabl CR , Neumann E |
| Ref : Biochemistry , 35 :685 , 1996 |
| Abstract : |
| PubMedSearch : Rosenberry_1996_Biochemistry_35_685 |
| PubMedID: 8547248 |
| Title : Fasciculin 2 binds to the peripheral site on acetylcholinesterase and inhibits substrate hydrolysis by slowing a step involving proton transfer during enzyme acylation - Eastman_1995_J.Biol.Chem_270_19694 |
| Author(s) : Eastman J , Wilson EJ , Cervenansky C , Rosenberry TL |
| Ref : Journal of Biological Chemistry , 270 :19694 , 1995 |
| Abstract : |
| PubMedSearch : Eastman_1995_J.Biol.Chem_270_19694 |
| PubMedID: 7649979 |
| Title : Contribution of aromatic moieties of tyrosine 133 and of the anionic subsite tryptophan 86 to catalytic efficiency and allosteric modulation of acetylcholinesterase - Ordentlich_1995_J.Biol.Chem_270_2082 |
| Author(s) : Ordentlich A , Barak D , Kronman C , Ariel N , Segall Y , Velan B , Shafferman A |
| Ref : Journal of Biological Chemistry , 270 :2082 , 1995 |
| Abstract : |
| PubMedSearch : Ordentlich_1995_J.Biol.Chem_270_2082 |
| PubMedID: 7836436 |
| Title : 6-Coumarin diazonium salt: a specific affinity label of the Torpedo acetylcholinesterase peripheral site - Schalk_1995_Mol.Pharmacol_48_1063 |
| Author(s) : Schalk I , Ehret-Sabatier L , Le Feuvre Y , Bon S , Massoulie J , Goeldner M |
| Ref : Molecular Pharmacology , 48 :1063 , 1995 |
| Abstract : |
| PubMedSearch : Schalk_1995_Mol.Pharmacol_48_1063 |
| PubMedID: 8848006 |
| Title : Different effects of two peripheral anionic site-binding ligands on acetylcholinesterase active-site gorge topography revealed by electron paramagnetic resonance - Grubic_1995_Biochim.Biophys.Acta_1249_155 |
| Author(s) : Grubic Z , Stalc A , Sentjurc M , Pecar S , Gentry MK , Doctor BP |
| Ref : Biochimica & Biophysica Acta , 1249 :155 , 1995 |
| Abstract : |
| PubMedSearch : Grubic_1995_Biochim.Biophys.Acta_1249_155 |
| PubMedID: 7599168 |
| Title : Contribution of aromatic moieties of tyrosine 133 and of the anionic subsite tryptophan 86 to catalytic efficiency and allosteric modulation of acetylcholinesterase - Ordentlich_1995_J.Biol.Chem_270_2082 |
| Author(s) : Ordentlich A , Barak D , Kronman C , Ariel N , Segall Y , Velan B , Shafferman A |
| Ref : Journal of Biological Chemistry , 270 :2082 , 1995 |
| Abstract : |
| PubMedSearch : Ordentlich_1995_J.Biol.Chem_270_2082 |
| PubMedID: 7836436 |
| Title : Characterization of monoclonal antibodies that strongly inhibit Electrophorus electricus acetylcholinesterase - Remy_1995_Eur.J.Biochem_231_651 |
| Author(s) : Remy MH , Frobert Y , Grassi J |
| Ref : European Journal of Biochemistry , 231 :651 , 1995 |
| Abstract : |
| PubMedSearch : Remy_1995_Eur.J.Biochem_231_651 |
| PubMedID: 7649165 |
| Title : Characterization of monoclonal antibodies that strongly inhibit Electrophorus electricus acetylcholinesterase - Remy_1995_Eur.J.Biochem_231_651 |
| Author(s) : Remy MH , Frobert Y , Grassi J |
| Ref : European Journal of Biochemistry , 231 :651 , 1995 |
| Abstract : |
| PubMedSearch : Remy_1995_Eur.J.Biochem_231_651 |
| PubMedID: 7649165 |
| Title : Allosteric modulation of acetylcholinesterase activity by peripheral ligands involves a conformational transition of the anionic subsite - Barak_1995_Biochemistry_34_15444 |
| Author(s) : Barak D , Ordentlich A , Bromberg A , Kronman C , Marcus D , Lazar A , Ariel N , Velan B , Shafferman A |
| Ref : Biochemistry , 34 :15444 , 1995 |
| Abstract : |
| PubMedSearch : Barak_1995_Biochemistry_34_15444 |
| PubMedID: 7492545 |
| Title : Fasciculin 2 binds to the peripheral site on acetylcholinesterase and inhibits substrate hydrolysis by slowing a step involving proton transfer during enzyme acylation - Eastman_1995_J.Biol.Chem_270_19694 |
| Author(s) : Eastman J , Wilson EJ , Cervenansky C , Rosenberry TL |
| Ref : Journal of Biological Chemistry , 270 :19694 , 1995 |
| Abstract : |
| PubMedSearch : Eastman_1995_J.Biol.Chem_270_19694 |
| PubMedID: 7649979 |
| Title : 6-Coumarin diazonium salt: a specific affinity label of the Torpedo acetylcholinesterase peripheral site - Schalk_1995_Mol.Pharmacol_48_1063 |
| Author(s) : Schalk I , Ehret-Sabatier L , Le Feuvre Y , Bon S , Massoulie J , Goeldner M |
| Ref : Molecular Pharmacology , 48 :1063 , 1995 |
| Abstract : |
| PubMedSearch : Schalk_1995_Mol.Pharmacol_48_1063 |
| PubMedID: 8848006 |
| Title : Different effects of two peripheral anionic site-binding ligands on acetylcholinesterase active-site gorge topography revealed by electron paramagnetic resonance - Grubic_1995_Biochim.Biophys.Acta_1249_155 |
| Author(s) : Grubic Z , Stalc A , Sentjurc M , Pecar S , Gentry MK , Doctor BP |
| Ref : Biochimica & Biophysica Acta , 1249 :155 , 1995 |
| Abstract : |
| PubMedSearch : Grubic_1995_Biochim.Biophys.Acta_1249_155 |
| PubMedID: 7599168 |
| Title : Different effects of two peripheral anionic site-binding ligands on acetylcholinesterase active-site gorge topography revealed by electron paramagnetic resonance - Grubic_1995_Biochim.Biophys.Acta_1249_155 |
| Author(s) : Grubic Z , Stalc A , Sentjurc M , Pecar S , Gentry MK , Doctor BP |
| Ref : Biochimica & Biophysica Acta , 1249 :155 , 1995 |
| Abstract : |
| PubMedSearch : Grubic_1995_Biochim.Biophys.Acta_1249_155 |
| PubMedID: 7599168 |
| Title : Characterization of monoclonal antibodies that strongly inhibit Electrophorus electricus acetylcholinesterase - Remy_1995_Eur.J.Biochem_231_651 |
| Author(s) : Remy MH , Frobert Y , Grassi J |
| Ref : European Journal of Biochemistry , 231 :651 , 1995 |
| Abstract : |
| PubMedSearch : Remy_1995_Eur.J.Biochem_231_651 |
| PubMedID: 7649165 |
| Title : Contribution of aromatic moieties of tyrosine 133 and of the anionic subsite tryptophan 86 to catalytic efficiency and allosteric modulation of acetylcholinesterase - Ordentlich_1995_J.Biol.Chem_270_2082 |
| Author(s) : Ordentlich A , Barak D , Kronman C , Ariel N , Segall Y , Velan B , Shafferman A |
| Ref : Journal of Biological Chemistry , 270 :2082 , 1995 |
| Abstract : |
| PubMedSearch : Ordentlich_1995_J.Biol.Chem_270_2082 |
| PubMedID: 7836436 |
| Title : Allosteric modulation of acetylcholinesterase activity by peripheral ligands involves a conformational transition of the anionic subsite - Barak_1995_Biochemistry_34_15444 |
| Author(s) : Barak D , Ordentlich A , Bromberg A , Kronman C , Marcus D , Lazar A , Ariel N , Velan B , Shafferman A |
| Ref : Biochemistry , 34 :15444 , 1995 |
| Abstract : |
| PubMedSearch : Barak_1995_Biochemistry_34_15444 |
| PubMedID: 7492545 |
| Title : Fasciculin 2 binds to the peripheral site on acetylcholinesterase and inhibits substrate hydrolysis by slowing a step involving proton transfer during enzyme acylation - Eastman_1995_J.Biol.Chem_270_19694 |
| Author(s) : Eastman J , Wilson EJ , Cervenansky C , Rosenberry TL |
| Ref : Journal of Biological Chemistry , 270 :19694 , 1995 |
| Abstract : |
| PubMedSearch : Eastman_1995_J.Biol.Chem_270_19694 |
| PubMedID: 7649979 |
| Title : 6-Coumarin diazonium salt: a specific affinity label of the Torpedo acetylcholinesterase peripheral site - Schalk_1995_Mol.Pharmacol_48_1063 |
| Author(s) : Schalk I , Ehret-Sabatier L , Le Feuvre Y , Bon S , Massoulie J , Goeldner M |
| Ref : Molecular Pharmacology , 48 :1063 , 1995 |
| Abstract : |
| PubMedSearch : Schalk_1995_Mol.Pharmacol_48_1063 |
| PubMedID: 8848006 |
| Title : Allosteric modulation of acetylcholinesterase activity by peripheral ligands involves a conformational transition of the anionic subsite - Barak_1995_Biochemistry_34_15444 |
| Author(s) : Barak D , Ordentlich A , Bromberg A , Kronman C , Marcus D , Lazar A , Ariel N , Velan B , Shafferman A |
| Ref : Biochemistry , 34 :15444 , 1995 |
| Abstract : |
| PubMedSearch : Barak_1995_Biochemistry_34_15444 |
| PubMedID: 7492545 |
| Title : Differential effects of peripheral site ligands on Torpedo and chicken acetylcholinesterase - Eichler_1994_Mol.Pharmacol_45_335 |
| Author(s) : Eichler J , Anselmet A , Sussman JL , Massoulie J , Silman I |
| Ref : Molecular Pharmacology , 45 :335 , 1994 |
| Abstract : |
| PubMedSearch : Eichler_1994_Mol.Pharmacol_45_335 |
| PubMedID: 8114681 |
| Title : Acetylcholinesterase peripheral anionic site degeneracy conferred by amino acid arrays sharing a common core - Barak_1994_J.Biol.Chem_269_6296 |
| Author(s) : Barak D , Kronman C , Ordentlich A , Ariel N , Bromberg A , Marcus D , Lazar A , Velan B , Shafferman A |
| Ref : Journal of Biological Chemistry , 269 :6296 , 1994 |
| Abstract : |
| PubMedSearch : Barak_1994_J.Biol.Chem_269_6296 |
| PubMedID: 8119978 |
| Title : Differential effects of peripheral site ligands on Torpedo and chicken acetylcholinesterase - Eichler_1994_Mol.Pharmacol_45_335 |
| Author(s) : Eichler J , Anselmet A , Sussman JL , Massoulie J , Silman I |
| Ref : Molecular Pharmacology , 45 :335 , 1994 |
| Abstract : |
| PubMedSearch : Eichler_1994_Mol.Pharmacol_45_335 |
| PubMedID: 8114681 |
| Title : Acetylcholinesterase peripheral anionic site degeneracy conferred by amino acid arrays sharing a common core - Barak_1994_J.Biol.Chem_269_6296 |
| Author(s) : Barak D , Kronman C , Ordentlich A , Ariel N , Bromberg A , Marcus D , Lazar A , Velan B , Shafferman A |
| Ref : Journal of Biological Chemistry , 269 :6296 , 1994 |
| Abstract : |
| PubMedSearch : Barak_1994_J.Biol.Chem_269_6296 |
| PubMedID: 8119978 |
| Title : Acetylcholinesterase peripheral anionic site degeneracy conferred by amino acid arrays sharing a common core - Barak_1994_J.Biol.Chem_269_6296 |
| Author(s) : Barak D , Kronman C , Ordentlich A , Ariel N , Bromberg A , Marcus D , Lazar A , Velan B , Shafferman A |
| Ref : Journal of Biological Chemistry , 269 :6296 , 1994 |
| Abstract : |
| PubMedSearch : Barak_1994_J.Biol.Chem_269_6296 |
| PubMedID: 8119978 |
| Title : Differential effects of peripheral site ligands on Torpedo and chicken acetylcholinesterase - Eichler_1994_Mol.Pharmacol_45_335 |
| Author(s) : Eichler J , Anselmet A , Sussman JL , Massoulie J , Silman I |
| Ref : Molecular Pharmacology , 45 :335 , 1994 |
| Abstract : |
| PubMedSearch : Eichler_1994_Mol.Pharmacol_45_335 |
| PubMedID: 8114681 |
| Title : Binding of 125I-fasciculin to rat brain acetylcholinesterase. The complex still binds diisopropyl fluorophosphate - Marchot_1993_J.Biol.Chem_268_12458 |
| Author(s) : Marchot P , Khelif A , Ji YH , Mansuelle P , Bougis PE |
| Ref : Journal of Biological Chemistry , 268 :12458 , 1993 |
| Abstract : |
| PubMedSearch : Marchot_1993_J.Biol.Chem_268_12458 |
| PubMedID: 8509385 |
| Title : Binding of 125I-fasciculin to rat brain acetylcholinesterase. The complex still binds diisopropyl fluorophosphate - Marchot_1993_J.Biol.Chem_268_12458 |
| Author(s) : Marchot P , Khelif A , Ji YH , Mansuelle P , Bougis PE |
| Ref : Journal of Biological Chemistry , 268 :12458 , 1993 |
| Abstract : |
| PubMedSearch : Marchot_1993_J.Biol.Chem_268_12458 |
| PubMedID: 8509385 |
| Title : Binding of 125I-fasciculin to rat brain acetylcholinesterase. The complex still binds diisopropyl fluorophosphate - Marchot_1993_J.Biol.Chem_268_12458 |
| Author(s) : Marchot P , Khelif A , Ji YH , Mansuelle P , Bougis PE |
| Ref : Journal of Biological Chemistry , 268 :12458 , 1993 |
| Abstract : |
| PubMedSearch : Marchot_1993_J.Biol.Chem_268_12458 |
| PubMedID: 8509385 |
| Title : Substrate inhibition of acetylcholinesterase: residues affecting signal transduction from the surface to the catalytic center - Shafferman_1992_EMBO.J_11_3561 |
| Author(s) : Shafferman A , Velan B , Ordentlich A , Kronman C , Grosfeld H , Leitner M , Flashner Y , Cohen S , Barak D , Ariel N |
| Ref : EMBO Journal , 11 :3561 , 1992 |
| Abstract : |
| PubMedSearch : Shafferman_1992_EMBO.J_11_3561 |
| PubMedID: 1396557 |
| Title : Substrate inhibition of acetylcholinesterase: residues affecting signal transduction from the surface to the catalytic center - Shafferman_1992_EMBO.J_11_3561 |
| Author(s) : Shafferman A , Velan B , Ordentlich A , Kronman C , Grosfeld H , Leitner M , Flashner Y , Cohen S , Barak D , Ariel N |
| Ref : EMBO Journal , 11 :3561 , 1992 |
| Abstract : |
| PubMedSearch : Shafferman_1992_EMBO.J_11_3561 |
| PubMedID: 1396557 |
| Title : Substrate inhibition of acetylcholinesterase: residues affecting signal transduction from the surface to the catalytic center - Shafferman_1992_EMBO.J_11_3561 |
| Author(s) : Shafferman A , Velan B , Ordentlich A , Kronman C , Grosfeld H , Leitner M , Flashner Y , Cohen S , Barak D , Ariel N |
| Ref : EMBO Journal , 11 :3561 , 1992 |
| Abstract : |
| PubMedSearch : Shafferman_1992_EMBO.J_11_3561 |
| PubMedID: 1396557 |
| Title : Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding - Taylor_1975_Biochemistry_14_1989 |
| Author(s) : Taylor P , Lappi S |
| Ref : Biochemistry , 14 :1989 , 1975 |
| Abstract : |
| PubMedSearch : Taylor_1975_Biochemistry_14_1989 |
| PubMedID: 1125207 |
| Title : Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding - Taylor_1975_Biochemistry_14_1989 |
| Author(s) : Taylor P , Lappi S |
| Ref : Biochemistry , 14 :1989 , 1975 |
| Abstract : |
| PubMedSearch : Taylor_1975_Biochemistry_14_1989 |
| PubMedID: 1125207 |
| Title : Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding - Taylor_1975_Biochemistry_14_1989 |
| Author(s) : Taylor P , Lappi S |
| Ref : Biochemistry , 14 :1989 , 1975 |
| Abstract : |
| PubMedSearch : Taylor_1975_Biochemistry_14_1989 |
| PubMedID: 1125207 |