Paraoxon
Pesticide. Results from the metabolism in vivo of Parathion (oxygen replaces the sulfur atom), Often used in biochemical investigations of serine hydrolases (structures of not alpha/beta hydrolases includes SGNH 1J00)
General
Type : Organophosphate,Insecticide,Derivative of Paraoxon,pNP
Chemical_Nomenclature : diethyl (4-nitrophenyl) phosphate
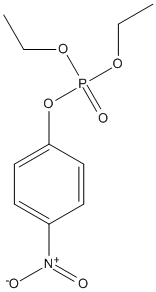
Canonical SMILES : CCOP(=O)(OCC)OC1=CC=C(C=C1)[N+](=O)[O-]
InChI : InChI=1S\/C10H14NO6P\/c1-3-15-18(14,16-4-2)17-10-7-5-9(6-8-10)11(12)13\/h5-8H,3-4H2,1-2H3
InChIKey : WYMSBXTXOHUIGT-UHFFFAOYSA-N
Other name(s) : phosphoric acid diethyl 4-nitrophenyl ester,Diethyl 4-nitrophenyl phosphate,diethyl p-nitrophenyl phosphate,phosphacol,E600,ester25,eticol,phosphakol,nintacol,miotisal A,solumlaucit,Mintacol,Fosfakol,Ethyl paraoxon,DNP,Diethyl paraoxon,paraoxon-ethyl,paraoxon ethyl
MW : 275.21
Formula : C10H14NO6P
CAS_number : 311-45-5
PubChem : 9395
UniChem : WYMSBXTXOHUIGT-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Paraoxon
Target
Families : Paraoxon ligand of proteins in family: BCHE || ACHE || Cutinase || Bacterial_lip_FamI.1 || A85-EsteraseD-FGH || Acetyl-esterase_deacetylase || Hormone-sensitive_lipase_like || PAF-Acetylhydrolase || CarbLipBact_1 || Pancreatic_lipase || 5_AlphaBeta_hydrolase || Bacterial_lip_FamI.3 || Plant_carboxylesterase || A85-Mycolyl-transferase || Lipase_3 || Lipase_2 || Dienelactone_hydrolase
Stucture :
References (58)
| Title : Structure and Mechanism of a Cold-Adapted Bacterial Lipase - van der Ent_2022_Biochemistry__ |
| Author(s) : van der Ent F , Lund BA , Svalberg L , Purg M , Chukwu G , Widersten M , Isaksen GV , Brandsdal BO , qvist J |
| Ref : Biochemistry , : , 2022 |
| Abstract : van der Ent_2022_Biochemistry__ |
| ESTHER : van der Ent_2022_Biochemistry__ |
| PubMedSearch : van der Ent_2022_Biochemistry__ |
| PubMedID: 35503728 |
| Gene_locus related to this paper: bacpu-w8fke7 |
| Title : Ultrahigh-Throughput Directed Evolution of a Metal-Free alpha\/beta-Hydrolase with a Cys-His-Asp Triad into an Efficient Phosphotriesterase - Schnettler_2022_J.Am.Chem.Soc__ |
| Author(s) : Schnettler JD , Klein OJ , Kaminski TS , Colin PY , Hollfelder F |
| Ref : Journal of the American Chemical Society , : , 2022 |
| Abstract : Schnettler_2022_J.Am.Chem.Soc__ |
| ESTHER : Schnettler_2022_J.Am.Chem.Soc__ |
| PubMedSearch : Schnettler_2022_J.Am.Chem.Soc__ |
| PubMedID: 36583539 |
| Gene_locus related to this paper: 9bact-KP212148 |
| Title : Structural and dynamic effects of paraoxon binding to human acetylcholinesterase by X-ray crystallography and inelastic neutron scattering - Gerlits_2022_Structure_30_1538 |
| Author(s) : Gerlits O , Fajer M , Cheng X , Blumenthal DK , Radic Z , Kovalevsky A |
| Ref : Structure , 30 :1538 , 2022 |
| Abstract : Gerlits_2022_Structure_30_1538 |
| ESTHER : Gerlits_2022_Structure_30_1538 |
| PubMedSearch : Gerlits_2022_Structure_30_1538 |
| PubMedID: 36265484 |
| Gene_locus related to this paper: human-ACHE |
| Title : Covalent inhibition of hAChE by organophosphates causes homodimer dissociation through long-range allosteric effects - Blumenthal_2021_J.Biol.Chem__ |
| Author(s) : Blumenthal DK , Cheng X , Fajer M , Ho KY , Rohrer J , Gerlits O , Taylor P , Juneja P , Kovalevsky A , Radic Z |
| Ref : Journal of Biological Chemistry , :101007 , 2021 |
| Abstract : Blumenthal_2021_J.Biol.Chem__ |
| ESTHER : Blumenthal_2021_J.Biol.Chem__ |
| PubMedSearch : Blumenthal_2021_J.Biol.Chem__ |
| PubMedID: 34324828 |
| Gene_locus related to this paper: human-ACHE |
| Title : Principles of lipid-enzyme interactions in the limbus region of the catalytic site of Candida antarctica Lipase B - Silvestrini_2020_Int.J.Biol.Macromol_158_358 |
| Author(s) : Silvestrini L , Cianci M |
| Ref : Int J Biol Macromol , 158 :358 , 2020 |
| Abstract : Silvestrini_2020_Int.J.Biol.Macromol_158_358 |
| ESTHER : Silvestrini_2020_Int.J.Biol.Macromol_158_358 |
| PubMedSearch : Silvestrini_2020_Int.J.Biol.Macromol_158_358 |
| PubMedID: 32380114 |
| Gene_locus related to this paper: canar-LipB |
| Title : Functional and structural characterization of a thermostable acetyl esterase from Thermotoga maritima - Levisson_2012_Proteins_80_1545 |
| Author(s) : Levisson M , Han GW , Deller MC , Xu Q , Biely P , Hendriks S , Ten Eyck LF , Flensburg C , Roversi P , Miller MD , McMullan D , von Delft F , Kreusch A , Deacon AM , Van der Oost J , Lesley SA , Elsliger MA , Kengen SW , Wilson IA |
| Ref : Proteins , 80 :1545 , 2012 |
| Abstract : Levisson_2012_Proteins_80_1545 |
| ESTHER : Levisson_2012_Proteins_80_1545 |
| PubMedSearch : Levisson_2012_Proteins_80_1545 |
| PubMedID: 22411095 |
| Gene_locus related to this paper: thema-TM0077 |
| Title : Structure and stability of a thermostable carboxylesterase from the thermoacidophilic archaeon Sulfolobus tokodaii. - Angkawidjaja_2012_Febs.J_279_3071 |
| Author(s) : Angkawidjaja C , Koga Y , Takano K , Kanaya S |
| Ref : Febs J , 279 :3071 , 2012 |
| Abstract : Angkawidjaja_2012_Febs.J_279_3071 |
| ESTHER : Angkawidjaja_2012_Febs.J_279_3071 |
| PubMedSearch : Angkawidjaja_2012_Febs.J_279_3071 |
| PubMedID: 22748144 |
| Gene_locus related to this paper: sulto-ST0071 |
| Title : The crystal structure of the cephalosporin deacetylating enzyme acetyl xylan esterase bound to paraoxon explains the low sensitivity of this serine hydrolase to organophosphate inactivation - Montoro-Garcia_2011_Biochem.J_436_321 |
| Author(s) : Montoro-Garcia S , Gil-Ortiz F , Garcia-Carmona F , Polo LM , Rubio V , Sanchez-Ferrer A |
| Ref : Biochemical Journal , 436 :321 , 2011 |
| Abstract : Montoro-Garcia_2011_Biochem.J_436_321 |
| ESTHER : Montoro-Garcia_2011_Biochem.J_436_321 |
| PubMedSearch : Montoro-Garcia_2011_Biochem.J_436_321 |
| PubMedID: 21382014 |
| Gene_locus related to this paper: bacpu-AXE |
| Title : X-ray crystallographic and MD simulation studies on the mechanism of interfacial activation of a family I.3 lipase with two lids - Angkawidjaja_2010_J.Mol.Biol_400_82 |
| Author(s) : Angkawidjaja C , Matsumura H , Koga Y , Takano K , Kanaya S |
| Ref : Journal of Molecular Biology , 400 :82 , 2010 |
| Abstract : Angkawidjaja_2010_J.Mol.Biol_400_82 |
| ESTHER : Angkawidjaja_2010_J.Mol.Biol_400_82 |
| PubMedSearch : Angkawidjaja_2010_J.Mol.Biol_400_82 |
| PubMedID: 20438738 |
| Gene_locus related to this paper: psesp-Q9RBY1 |
| Title : Crystal structure of human plasma platelet-activating factor acetylhydrolase: structural implication to lipoprotein binding and catalysis - Samanta_2008_J.Biol.Chem_283_31617 |
| Author(s) : Samanta U , Bahnson BJ |
| Ref : Journal of Biological Chemistry , 283 :31617 , 2008 |
| Abstract : Samanta_2008_J.Biol.Chem_283_31617 |
| ESTHER : Samanta_2008_J.Biol.Chem_283_31617 |
| PubMedSearch : Samanta_2008_J.Biol.Chem_283_31617 |
| PubMedID: 18784071 |
| Gene_locus related to this paper: human-PLA2G7 |
| Title : High-resolution crystal structure of plant carboxylesterase AeCXE1, from Actinidia eriantha, and its complex with a high-affinity inhibitor paraoxon - Ileperuma_2007_Biochemistry_46_1851 |
| Author(s) : Ileperuma NR , Marshall SD , Squire CJ , Baker HM , Oakeshott JG , Russell RJ , Plummer KM , Newcomb RD , Baker EN |
| Ref : Biochemistry , 46 :1851 , 2007 |
| Abstract : Ileperuma_2007_Biochemistry_46_1851 |
| ESTHER : Ileperuma_2007_Biochemistry_46_1851 |
| PubMedSearch : Ileperuma_2007_Biochemistry_46_1851 |
| PubMedID: 17256879 |
| Gene_locus related to this paper: actde-CXE1 |
| Title : Further biochemical characterization of human pancreatic lipase-related protein 2 expressed in yeast cells - Eydoux_2007_J.Lipid.Res_48_1539 |
| Author(s) : Eydoux C , De Caro J , Ferrato F , Boullanger P , Lafont D , Laugier R , Carriere F , de Caro A |
| Ref : J Lipid Res , 48 :1539 , 2007 |
| Abstract : Eydoux_2007_J.Lipid.Res_48_1539 |
| ESTHER : Eydoux_2007_J.Lipid.Res_48_1539 |
| PubMedSearch : Eydoux_2007_J.Lipid.Res_48_1539 |
| PubMedID: 17401110 |
| Gene_locus related to this paper: human-PNLIPRP2 |
| Title : Mutagenesis of organophosphorus hydrolase to enhance hydrolysis of the nerve agent VX - Gopal_2000_Biochem.Biophys.Res.Commun_279_516 |
| Author(s) : Gopal S , Rastogi V , Ashman W , Mulbry W |
| Ref : Biochemical & Biophysical Research Communications , 279 :516 , 2000 |
| Abstract : Gopal_2000_Biochem.Biophys.Res.Commun_279_516 |
| ESTHER : Gopal_2000_Biochem.Biophys.Res.Commun_279_516 |
| PubMedSearch : Gopal_2000_Biochem.Biophys.Res.Commun_279_516 |
| PubMedID: 11118318 |
| Title : Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines - Ronning_2000_Nat.Struct.Biol_7_141 |
| Author(s) : Ronning DR , Klabunde T , Besra GS , Vissa VD , Belisle JT , Sacchettini JC |
| Ref : Nat Struct Biol , 7 :141 , 2000 |
| Abstract : Ronning_2000_Nat.Struct.Biol_7_141 |
| ESTHER : Ronning_2000_Nat.Struct.Biol_7_141 |
| PubMedSearch : Ronning_2000_Nat.Struct.Biol_7_141 |
| PubMedID: 10655617 |
| Gene_locus related to this paper: myctu-a85c |
| Title : Stereochemical constraints on the substrate specificity of phosphotriesterase - Hong_1999_Biochemistry_38_1159 |
| Author(s) : Hong SB , Raushel FM |
| Ref : Biochemistry , 38 :1159 , 1999 |
| Abstract : Hong_1999_Biochemistry_38_1159 |
| ESTHER : Hong_1999_Biochemistry_38_1159 |
| PubMedSearch : Hong_1999_Biochemistry_38_1159 |
| PubMedID: 9930975 |
| Title : Stereochemical preferences for chiral substrates by the bacterial phosphotriesterase - Hong_1999_Chem.Biol.Interact_119-120_225 |
| Author(s) : Hong SB , Raushel FM |
| Ref : Chemico-Biological Interactions , 119-120 :225 , 1999 |
| Abstract : Hong_1999_Chem.Biol.Interact_119-120_225 |
| ESTHER : Hong_1999_Chem.Biol.Interact_119-120_225 |
| PubMedSearch : Hong_1999_Chem.Biol.Interact_119-120_225 |
| PubMedID: 10421456 |
| Title : Inhibition of carboxylesterases in SH-SY5Y human and NB41A3 mouse neuroblastoma cells by organophosphorus esters - Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| Author(s) : Ehrich M , Correll L |
| Ref : J Toxicol Environ Health , 53 :385 , 1998 |
| Abstract : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| ESTHER : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| PubMedSearch : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| PubMedID: 9515941 |
| Title : Enzyme-based assay for quantification of paraoxon in blood of parathion poisoned patients - Eyer_1998_Hum.Exp.Toxicol_17_645 |
| Author(s) : Eyer F , Eyer P |
| Ref : Hum Exp Toxicol , 17 :645 , 1998 |
| Abstract : Eyer_1998_Hum.Exp.Toxicol_17_645 |
| ESTHER : Eyer_1998_Hum.Exp.Toxicol_17_645 |
| PubMedSearch : Eyer_1998_Hum.Exp.Toxicol_17_645 |
| PubMedID: 9988368 |
| Title : Effect of phenobarbital and beta-naphthoflavone on activities of different rat esterases after paraoxon exposure - Kaliste-Korhonen_1998_Gen.Pharmacol_31_307 |
| Author(s) : Kaliste-Korhonen E , Tuovinen K , Hanninen O |
| Ref : General Pharmacology , 31 :307 , 1998 |
| Abstract : Kaliste-Korhonen_1998_Gen.Pharmacol_31_307 |
| ESTHER : Kaliste-Korhonen_1998_Gen.Pharmacol_31_307 |
| PubMedSearch : Kaliste-Korhonen_1998_Gen.Pharmacol_31_307 |
| PubMedID: 9688478 |
| Title : Augmented hydrolysis of diisopropyl fluorophosphate in engineered mutants of phosphotriesterase - Watkins_1997_J.Biol.Chem_272_25596 |
| Author(s) : Watkins LM , Mahoney HJ , McCulloch JK , Raushel FM |
| Ref : Journal of Biological Chemistry , 272 :25596 , 1997 |
| Abstract : Watkins_1997_J.Biol.Chem_272_25596 |
| ESTHER : Watkins_1997_J.Biol.Chem_272_25596 |
| PubMedSearch : Watkins_1997_J.Biol.Chem_272_25596 |
| PubMedID: 9325279 |
| Title : Importance of aspartate-70 in organophosphate inhibition, oxime re-activation and aging of human butyrylcholinesterase - Masson_1997_Biochem.J_325_53 |
| Author(s) : Masson P , Froment MT , Bartels CF , Lockridge O |
| Ref : Biochemical Journal , 325 :53 , 1997 |
| Abstract : Masson_1997_Biochem.J_325_53 |
| ESTHER : Masson_1997_Biochem.J_325_53 |
| PubMedSearch : Masson_1997_Biochem.J_325_53 |
| PubMedID: 9224629 |
| Title : A physiologically based pharmacokinetic and pharmacodynamic model for paraoxon in rainbow trout - Abbas_1997_Toxicol.Appl.Pharmacol_145_192 |
| Author(s) : Abbas R , Hayton WL |
| Ref : Toxicol Appl Pharmacol , 145 :192 , 1997 |
| Abstract : Abbas_1997_Toxicol.Appl.Pharmacol_145_192 |
| ESTHER : Abbas_1997_Toxicol.Appl.Pharmacol_145_192 |
| PubMedSearch : Abbas_1997_Toxicol.Appl.Pharmacol_145_192 |
| PubMedID: 9221837 |
| Title : Dose-dependent inhibition of phospholipase A2 by paraoxon in vitro: preliminary results - Petroianu_1997_J.Appl.Toxicol_17_421 |
| Author(s) : Petroianu GA , Helfrich U , Schmitt A , Bergler W , Rufer R |
| Ref : Journal of Applied Toxicology , 17 :421 , 1997 |
| Abstract : Petroianu_1997_J.Appl.Toxicol_17_421 |
| ESTHER : Petroianu_1997_J.Appl.Toxicol_17_421 |
| PubMedSearch : Petroianu_1997_J.Appl.Toxicol_17_421 |
| PubMedID: 9418952 |
| Title : Paraoxon: cholinesterase-independent stimulation of transmitter release and selective block of ligand-gated ion channels in cultured hippocampal neurons - Rocha_1996_J.Pharmacol.Exp.Ther_278_1175 |
| Author(s) : Rocha ES , Swanson KL , Aracava Y , Goolsby JE , Maelicke A , Albuquerque EX |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 278 :1175 , 1996 |
| Abstract : Rocha_1996_J.Pharmacol.Exp.Ther_278_1175 |
| ESTHER : Rocha_1996_J.Pharmacol.Exp.Ther_278_1175 |
| PubMedSearch : Rocha_1996_J.Pharmacol.Exp.Ther_278_1175 |
| PubMedID: 8819500 |
| Title : Identification and isolation of two rat serum proteins with A-esterase activity toward paraoxon and chlorpyrifos-oxon - Pond_1996_Biochem.Pharmacol_52_363 |
| Author(s) : Pond AL , Coyne CP , Chambers HW , Chambers JE |
| Ref : Biochemical Pharmacology , 52 :363 , 1996 |
| Abstract : Pond_1996_Biochem.Pharmacol_52_363 |
| ESTHER : Pond_1996_Biochem.Pharmacol_52_363 |
| PubMedSearch : Pond_1996_Biochem.Pharmacol_52_363 |
| PubMedID: 8694862 |
| Title : Comparison of an Esterase Associated with Organophosphate Resistance inLucilia cuprina with an Orthologue Not Associated with Resistance in Drosophila melanogaster - Parker_1996_Pestic.Biochem.Physiol_55_85 |
| Author(s) : Parker AG , Campbell PM , Spackman ME , Russell RJ , Oakeshott JG |
| Ref : Pesticide Biochemistry and Physiology , 55 :85 , 1996 |
| Abstract : Parker_1996_Pestic.Biochem.Physiol_55_85 |
| ESTHER : Parker_1996_Pestic.Biochem.Physiol_55_85 |
| PubMedSearch : Parker_1996_Pestic.Biochem.Physiol_55_85 |
| PubMedID: 8980033 |
| Title : Kinetic analysis of the in vitro inhibition, aging, and reactivation of brain acetylcholinesterase from rat and channel catfish by paraoxon and chlorpyrifos-oxon - Carr_1996_Toxicol.Appl.Pharmacol_139_365 |
| Author(s) : Carr RL , Chambers JE |
| Ref : Toxicology & Applied Pharmacology , 139 :365 , 1996 |
| Abstract : Carr_1996_Toxicol.Appl.Pharmacol_139_365 |
| ESTHER : Carr_1996_Toxicol.Appl.Pharmacol_139_365 |
| PubMedSearch : Carr_1996_Toxicol.Appl.Pharmacol_139_365 |
| PubMedID: 8806854 |
| Title : Antagonism of the lethal effects of paraoxon by carrier erythrocytes containing phosphotriesterase - Pei_1995_Fundam.Appl.Toxicol_28_209 |
| Author(s) : Pei L , Petrikovics I , Way JL |
| Ref : Fundamental & Applied Toxicology , 28 :209 , 1995 |
| Abstract : Pei_1995_Fundam.Appl.Toxicol_28_209 |
| ESTHER : Pei_1995_Fundam.Appl.Toxicol_28_209 |
| PubMedSearch : Pei_1995_Fundam.Appl.Toxicol_28_209 |
| PubMedID: 8835230 |
| Title : Effect of externally added carnitine on the synthesis of acetylcholine in rat cerebral cortex cells - Wawrzenczyk_1995_Neurochem.Int_26_635 |
| Author(s) : Wawrzenczyk A , Nalecz KA , Nalecz MJ |
| Ref : Neurochem Int , 26 :635 , 1995 |
| Abstract : Wawrzenczyk_1995_Neurochem.Int_26_635 |
| ESTHER : Wawrzenczyk_1995_Neurochem.Int_26_635 |
| PubMedSearch : Wawrzenczyk_1995_Neurochem.Int_26_635 |
| PubMedID: 7670366 |
| Title : Raman spectroscopic study of conjugates of butyrylcholinesterase with organophosphates - Aslanian_1995_Biochim.Biophys.Acta_1249_37 |
| Author(s) : Aslanian D , Grof P , Renault F , Masson P |
| Ref : Biochimica & Biophysica Acta , 1249 :37 , 1995 |
| Abstract : Aslanian_1995_Biochim.Biophys.Acta_1249_37 |
| ESTHER : Aslanian_1995_Biochim.Biophys.Acta_1249_37 |
| PubMedSearch : Aslanian_1995_Biochim.Biophys.Acta_1249_37 |
| PubMedID: 7766682 |
| Title : Inhibition and aging of channel catfish brain acetylcholinesterase following exposure to two phosphorothionate insecticides and their active metabolites - Carr_1995_J.Toxicol.Env.Health_45_325 |
| Author(s) : Carr RL , Straus DL , Chambers JE |
| Ref : Journal of Toxicology & Environmental Health , 45 :325 , 1995 |
| Abstract : Carr_1995_J.Toxicol.Env.Health_45_325 |
| ESTHER : Carr_1995_J.Toxicol.Env.Health_45_325 |
| PubMedSearch : Carr_1995_J.Toxicol.Env.Health_45_325 |
| PubMedID: 7541841 |
| Title : Choline derivatives and sodium fluoride protect acetylcholinesterase against irreversible inhibition and aging by DFP and paraoxon - Dehlawi_1994_J.Biochem.Toxicol_9_261 |
| Author(s) : Dehlawi MS , Eldefrawi AT , Eldefrawi ME , Anis NA , Valdes JJ |
| Ref : Journal of Biochemical Toxicology , 9 :261 , 1994 |
| Abstract : Dehlawi_1994_J.Biochem.Toxicol_9_261 |
| ESTHER : Dehlawi_1994_J.Biochem.Toxicol_9_261 |
| PubMedSearch : Dehlawi_1994_J.Biochem.Toxicol_9_261 |
| PubMedID: 7853361 |
| Title : Phosphotriesterase--a promising candidate for use in detoxification of organophosphates - Tuovinen_1994_Fundam.Appl.Toxicol_23_578 |
| Author(s) : Tuovinen K , Kaliste-Korhonen E , Raushel FM , Hanninen O |
| Ref : Fundamental & Applied Toxicology , 23 :578 , 1994 |
| Abstract : Tuovinen_1994_Fundam.Appl.Toxicol_23_578 |
| ESTHER : Tuovinen_1994_Fundam.Appl.Toxicol_23_578 |
| PubMedSearch : Tuovinen_1994_Fundam.Appl.Toxicol_23_578 |
| PubMedID: 7867909 |
| Title : Cutinase, a lipolytic enzyme with a preformed oxyanion hole - Martinez_1994_Biochemistry_33_83 |
| Author(s) : Martinez C , Nicolas A , van Tilbeurgh H , Egloff MP , Cudrey C , Verger R , Cambillau C |
| Ref : Biochemistry , 33 :83 , 1994 |
| Abstract : Martinez_1994_Biochemistry_33_83 |
| ESTHER : Martinez_1994_Biochemistry_33_83 |
| PubMedSearch : Martinez_1994_Biochemistry_33_83 |
| PubMedID: 8286366 |
| Gene_locus related to this paper: fusso-cutas , orysa-LPL1 |
| Title : Carbamate poisoning and oxime treatment in children: a clinical and laboratory study - Lifshitz_1994_Pediatrics_93_652 |
| Author(s) : Lifshitz M , Rotenberg M , Sofer S , Tamiri T , Shahak E , Almog S |
| Ref : Pediatrics , 93 :652 , 1994 |
| Abstract : Lifshitz_1994_Pediatrics_93_652 |
| ESTHER : Lifshitz_1994_Pediatrics_93_652 |
| PubMedSearch : Lifshitz_1994_Pediatrics_93_652 |
| PubMedID: 8134223 |
| Title : Inhibition patterns of brain acetylcholinesterase and hepatic and plasma aliesterases following exposures to three phosphorothionate insecticides and their oxons in rats - Chambers_1993_Fundam.Appl.Toxicol_21_111 |
| Author(s) : Chambers JE , Carr RL |
| Ref : Fundamental & Applied Toxicology , 21 :111 , 1993 |
| Abstract : Chambers_1993_Fundam.Appl.Toxicol_21_111 |
| ESTHER : Chambers_1993_Fundam.Appl.Toxicol_21_111 |
| PubMedSearch : Chambers_1993_Fundam.Appl.Toxicol_21_111 |
| PubMedID: 7689992 |
| Title : Serum paraoxonase status: a major factor in determining resistance to organophosphates - Li_1993_J.Toxicol.Env.Health_40_337 |
| Author(s) : Li WF , Costa LG , Furlong CE |
| Ref : Journal of Toxicology & Environmental Health , 40 :337 , 1993 |
| Abstract : Li_1993_J.Toxicol.Env.Health_40_337 |
| ESTHER : Li_1993_J.Toxicol.Env.Health_40_337 |
| PubMedSearch : Li_1993_J.Toxicol.Env.Health_40_337 |
| PubMedID: 7693961 |
| Title : Cholinesterases of heart muscle. Characterization of multiple enzymes using kinetics of irreversible organophosphorus inhibition - Chemnitius_1992_Biochem.Pharmacol_43_823 |
| Author(s) : Chemnitius JM , Chemnitius GC , Haselmeyer KH , Kreuzer H , Zech R |
| Ref : Biochemical Pharmacology , 43 :823 , 1992 |
| Abstract : Chemnitius_1992_Biochem.Pharmacol_43_823 |
| ESTHER : Chemnitius_1992_Biochem.Pharmacol_43_823 |
| PubMedSearch : Chemnitius_1992_Biochem.Pharmacol_43_823 |
| PubMedID: 1540236 |
| Title : Determination of organophosphorous and carbamate insecticides by flow injection analysis - Kumaran_1992_Anal.Biochem_200_187 |
| Author(s) : Kumaran S , Tran-Minh C |
| Ref : Analytical Biochemistry , 200 :187 , 1992 |
| Abstract : Kumaran_1992_Anal.Biochem_200_187 |
| ESTHER : Kumaran_1992_Anal.Biochem_200_187 |
| PubMedSearch : Kumaran_1992_Anal.Biochem_200_187 |
| PubMedID: 1595894 |
| Title : Neurotoxicity of acute and repeated treatments of tabun, paraoxon, diisopropyl fluorophosphate and isofenphos to the hen - Henderson_1992_Toxicology_72_117 |
| Author(s) : Henderson JD , Higgins RJ , Dacre JC , Wilson BW |
| Ref : Toxicology , 72 :117 , 1992 |
| Abstract : Henderson_1992_Toxicology_72_117 |
| ESTHER : Henderson_1992_Toxicology_72_117 |
| PubMedSearch : Henderson_1992_Toxicology_72_117 |
| PubMedID: 1566275 |
| Title : Inactivation of gastric and pancreatic lipases by diethyl p-nitrophenyl phosphate - Moreau_1991_Biochemistry_30_1037 |
| Author(s) : Moreau H , Moulin A , Gargouri Y , Noel JP , Verger R |
| Ref : Biochemistry , 30 :1037 , 1991 |
| Abstract : Moreau_1991_Biochemistry_30_1037 |
| ESTHER : Moreau_1991_Biochemistry_30_1037 |
| PubMedSearch : Moreau_1991_Biochemistry_30_1037 |
| PubMedID: 1989675 |
| Gene_locus related to this paper: human-LIPF , human-PNLIP |
| Title : Putative M2 muscarinic receptors of rat heart have high affinity for organophosphorus anticholinesterases - Silveira_1990_Toxicol.Appl.Pharmacol_103_474 |
| Author(s) : Silveira CL , Eldefrawi AT , Eldefrawi ME |
| Ref : Toxicol Appl Pharmacol , 103 :474 , 1990 |
| Abstract : Silveira_1990_Toxicol.Appl.Pharmacol_103_474 |
| ESTHER : Silveira_1990_Toxicol.Appl.Pharmacol_103_474 |
| PubMedSearch : Silveira_1990_Toxicol.Appl.Pharmacol_103_474 |
| PubMedID: 2339420 |
| Title : [Catalytic properties of cholinesterases immobilized in a gelatin membrane] - Kuznetsova_1990_Ukr.Biokhim.Zh_62_42 |
| Author(s) : Kuznetsova LP , Kugusheva LI , Nikol'skaia EB |
| Ref : Ukr Biokhim Zh , 62 :42 , 1990 |
| Abstract : Kuznetsova_1990_Ukr.Biokhim.Zh_62_42 |
| ESTHER : Kuznetsova_1990_Ukr.Biokhim.Zh_62_42 |
| PubMedSearch : Kuznetsova_1990_Ukr.Biokhim.Zh_62_42 |
| PubMedID: 2087792 |
| Title : Short-term effects of paraoxon and atropine on schedule-controlled behavior in rats - Chambers_1989_Neurotoxicol.Teratol_11_427 |
| Author(s) : Chambers JE , Chambers HW |
| Ref : Neurotoxicology & Teratology , 11 :427 , 1989 |
| Abstract : Chambers_1989_Neurotoxicol.Teratol_11_427 |
| ESTHER : Chambers_1989_Neurotoxicol.Teratol_11_427 |
| PubMedSearch : Chambers_1989_Neurotoxicol.Teratol_11_427 |
| PubMedID: 2593981 |
| Title : Degradation by rat tissues in vitro of organophosphorus esters which inhibit cholinesterase - Pla_1989_Biochem.Pharmacol_38_1527 |
| Author(s) : Pla A , Johnson MK |
| Ref : Biochemical Pharmacology , 38 :1527 , 1989 |
| Abstract : Pla_1989_Biochem.Pharmacol_38_1527 |
| ESTHER : Pla_1989_Biochem.Pharmacol_38_1527 |
| PubMedSearch : Pla_1989_Biochem.Pharmacol_38_1527 |
| PubMedID: 2719724 |
| Title : A microassay-based procedure for measuring low levels of toxic organophosphorus compounds through acetylcholinesterase inhibition - Hammond_1989_Anal.Biochem_180_380 |
| Author(s) : Hammond PS , Forster JS |
| Ref : Analytical Biochemistry , 180 :380 , 1989 |
| Abstract : Hammond_1989_Anal.Biochem_180_380 |
| ESTHER : Hammond_1989_Anal.Biochem_180_380 |
| PubMedSearch : Hammond_1989_Anal.Biochem_180_380 |
| PubMedID: 2817369 |
| Title : Toxicity of an acute dose of agent VX and other organophosphorus esters in the chicken - Wilson_1988_J.Toxicol.Environ.Health_23_103 |
| Author(s) : Wilson BW , Henderson JD , Chow E , Schreider J , Goldman M , Culbertson R , Dacre JC |
| Ref : J Toxicol Environ Health , 23 :103 , 1988 |
| Abstract : Wilson_1988_J.Toxicol.Environ.Health_23_103 |
| ESTHER : Wilson_1988_J.Toxicol.Environ.Health_23_103 |
| PubMedSearch : Wilson_1988_J.Toxicol.Environ.Health_23_103 |
| PubMedID: 3336055 |
| Title : Effects of paraoxon, p-nitrophenol, phenyl saligenin cyclic phosphate, and phenol on the rat interleukin 2 system - Pruett_1988_Toxicol.Lett_40_11 |
| Author(s) : Pruett SB , Chambers JE |
| Ref : Toxicol Lett , 40 :11 , 1988 |
| Abstract : Pruett_1988_Toxicol.Lett_40_11 |
| ESTHER : Pruett_1988_Toxicol.Lett_40_11 |
| PubMedSearch : Pruett_1988_Toxicol.Lett_40_11 |
| PubMedID: 3257593 |
| Title : A esterases and their role in regulating the toxicity of organophosphates - Walker_1987_Arch.Toxicol_60_30 |
| Author(s) : Walker CH , Mackness MI |
| Ref : Archives of Toxicology , 60 :30 , 1987 |
| Abstract : Walker_1987_Arch.Toxicol_60_30 |
| ESTHER : Walker_1987_Arch.Toxicol_60_30 |
| PubMedSearch : Walker_1987_Arch.Toxicol_60_30 |
| PubMedID: 3304214 |
| Title : Studies on the toxicity, metabolism, and anticholinesterase properties of acephate and methamidophos - Hussain_1985_J.Env.Sci.Health_20_129 |
| Author(s) : Hussain MA , Mohamad RB , Oloffs PC |
| Ref : Journal of Environmental Science & Health Part B: Pesticides, Food Contaminants, & Agricultural Wastes , 20 :129 , 1985 |
| Abstract : Hussain_1985_J.Env.Sci.Health_20_129 |
| ESTHER : Hussain_1985_J.Env.Sci.Health_20_129 |
| PubMedSearch : Hussain_1985_J.Env.Sci.Health_20_129 |
| PubMedID: 3989221 |
| Title : Partial purification and properties of sheep serum A'-esterases - Mackness_1983_Biochem.Pharmacol_32_2291 |
| Author(s) : Mackness MI , Walker CH |
| Ref : Biochemical Pharmacology , 32 :2291 , 1983 |
| Abstract : Mackness_1983_Biochem.Pharmacol_32_2291 |
| ESTHER : Mackness_1983_Biochem.Pharmacol_32_2291 |
| PubMedSearch : Mackness_1983_Biochem.Pharmacol_32_2291 |
| PubMedID: 6309189 |
| Title : Brain cholinesterases. Differentiation of target enzymes for toxic organophosphorus compounds - Chemnitius_1983_Biochem.Pharmacol_32_1693 |
| Author(s) : Chemnitius JM , Haselmeyer KH , Zech R |
| Ref : Biochemical Pharmacology , 32 :1693 , 1983 |
| Abstract : Chemnitius_1983_Biochem.Pharmacol_32_1693 |
| ESTHER : Chemnitius_1983_Biochem.Pharmacol_32_1693 |
| PubMedSearch : Chemnitius_1983_Biochem.Pharmacol_32_1693 |
| PubMedID: 6870909 |
| Title : Characterization of the serine reacting with diethyl p-nitrophenyl phosphate in porcine pancreatic lipase - Guidoni_1981_Biochim.Biophys.Acta_660_148 |
| Author(s) : Guidoni A , Benkouka F , De Caro J , Rovery M |
| Ref : Biochimica & Biophysica Acta , 660 :148 , 1981 |
| Abstract : Guidoni_1981_Biochim.Biophys.Acta_660_148 |
| ESTHER : Guidoni_1981_Biochim.Biophys.Acta_660_148 |
| PubMedSearch : Guidoni_1981_Biochim.Biophys.Acta_660_148 |
| PubMedID: 6791692 |
| Gene_locus related to this paper: pig-1plip |
| Title : Evaluation of cytotoxic responses caused by selected organophosphorus esters in chick sympathetic ganglia cultures - Obersteiner_1978_Can.J.Comp.Med_42_80 |
| Author(s) : Obersteiner EJ , Sharma RP |
| Ref : Can J Comp Med , 42 :80 , 1978 |
| Abstract : Obersteiner_1978_Can.J.Comp.Med_42_80 |
| ESTHER : Obersteiner_1978_Can.J.Comp.Med_42_80 |
| PubMedSearch : Obersteiner_1978_Can.J.Comp.Med_42_80 |
| PubMedID: 565668 |
| Title : Inhibition of pancreatic lipase by mixed micelles of diethyl p-nitrophenyl phosphate and bile salts - Rouard_1978_Biochim.Biophys.Acta_530_227 |
| Author(s) : Rouard M , Sari H , Nurit S , Entressangles B , Desnuelle P |
| Ref : Biochimica & Biophysica Acta , 530 :227 , 1978 |
| Abstract : Rouard_1978_Biochim.Biophys.Acta_530_227 |
| ESTHER : Rouard_1978_Biochim.Biophys.Acta_530_227 |
| PubMedSearch : Rouard_1978_Biochim.Biophys.Acta_530_227 |
| PubMedID: 667092 |
| Gene_locus related to this paper: pig-1plip |
| Title : Phosphorylation of acetylcholinesterase in bovine blood by Paraoxon and Soman - Nenner_1973_Arch.Toxikol_30_87 |
| Author(s) : Nenner M |
| Ref : Arch Toxikol , 30 :87 , 1973 |
| Abstract : Nenner_1973_Arch.Toxikol_30_87 |
| ESTHER : Nenner_1973_Arch.Toxikol_30_87 |
| PubMedSearch : Nenner_1973_Arch.Toxikol_30_87 |
| PubMedID: 4685268 |
| Title : Mintacol (diethyl-p-nitrophenylphosphate) (Paraoxon) - |
| Author(s) : Augustinsson KB |
| Ref : Sven Farm Tidskr , 57 :261 , 1953 |
| PubMedID: 13077191 |
| Title : Enzyme studies with diethyl-p-nitrophenylphosphate (mintacol) - |
| Author(s) : Augustinsson KB |
| Ref : Acta Pharmacologica et Toxicologica (Copenh) , 9 :245 , 1953 |
| PubMedID: 13123943 |